less knowledge on regulatory strategy necessary
costs for initial certification saved
instead of 6 months
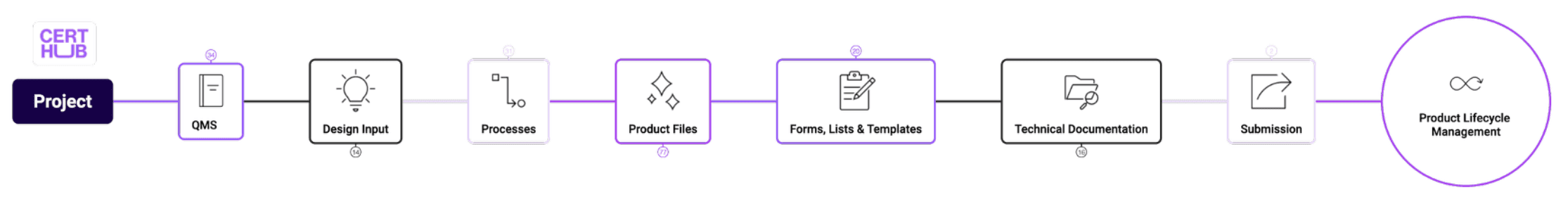
Traditional Medical Devices
Comprehensive QMS and technical documentation for Class I-III medical devices, covering design controls, risk management, clinical evaluation, and regulatory submissions.
ISO 13485 QMS Templates, EU MDR Technical Documentation, Risk Management (ISO 14971), Usability Engineering (IEC 62366)
60% faster regulatory submissions, 40% reduction in regulatory review cycles
In-Vitro Diagnostics
Specialized content for IVD manufacturers including analytical and clinical performance studies, quality control, and IVDR-specific requirements.
IVDR Compliance Templates, Performance Evaluation, Quality Control Procedures, Notified Body Submissions
50% reduction in IVDR transition costs, faster time-to-market for new diagnostics
Software as Medical Device
Complete framework for SaMD development, including software lifecycle processes, cybersecurity, AI/ML validation, and digital health regulations.
IEC 62304 Software Lifecycle, AI Act Compliance, Cybersecurity (ISO 14155), Digital Health Pathways
70% faster SaMD development cycles, future-proof AI Act readiness
© CertHub 2025